Abstract
INTRODUCTION: Allogeneic Mesenchymal stromal cell (MSC) infusion for refractory acute graft-versus-host disease (GVHD) is currently being evaluated in phase II and III clinical trials, in some of which our group has been involved. Nevertheless, some patients are not eligible for clinical trials and may be treated by compassionate use programs. Our objective was to analyze the results of the compassionate program in our institution to ascertain if the safety and efficacy of the treatment in this "real-life" setting is comparable or not to the published results of the phase II trials.
METHODS: We conducted a retrospective study of 43 patients who underwent allogeneic hematopoietic stem cell transplant (allo-SCT) and were treated with MSC by compassionate use in our center from June 2009 to date (August 2017). Allogeneic MSC were produced in our good manufacturing practice (GMP) cellular therapy facility and were obtained from third-party healthy donors. Twenty-eight days after the first infusion, the response and the adverse events were evaluated. In addition, the survival was assessed to date.
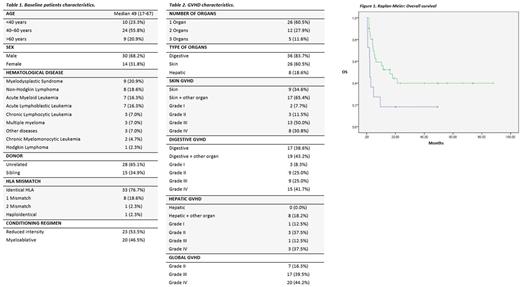
RESULTS: Patient characteristics are described in table 1, and GVHD characteristics are shown in table 2. Median time from diagnosis of GVHD to the first MSC infusion was 13 days (2 - 105), with a median number of prior treatment lines of 1 (range 1 to 4). Seventy six percent of infusions followed a sequential pattern (days 1, 4, 11 and 18), similar to the schedule of our clinical trials (Sánchez-Guijo F. Biol Blood Marrow Transplant 2014). The overall response rate (ORR) after 4 weeks was 72%, with complete response (CR) in 48%, and partial response (PR) in 23%. We did not see differences in responses by organ or by GVHD grade. Among responders, around 40% of patients relapsed with a median of time of 63 days (35 - 203). Median time at last follow-up was 164 days (15 - 2638) and in the group who achieved CR after cellular therapy 62% of patients were alive to date. Among non-responders, 83% of patients died due to GVHD. The median time of overall survival was 16 months (range 3 - 28) vs 2 months (range 1 - 3) in responders vs non-responders, respectively (p=0.018), as it is depicted in figure 1. Non-responders were 2.57 times more likely to die (95% CI, 1.14 - 5.83; p=0.023). Regarding safety, there were no adverse events reported such as infusion reactions or related infections.
CONCLUSIONS: MSC used for the treatment or refractory GVHD in "real-life" compassionate use programs seem to provide comparable results to published phase II trials, showing a safety profile, a high rate of responses and a potential benefit in survival.
Sanchez-Guijo: Incyte: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal